*Study implicates “arms race” between genes and germs*
Biologists have found new evidence of why mice, people and other vertebrate animals carry thousands of varieties of genes to make immune-system proteins named MHCs–even though some of those genes make vertebrate animals susceptible to infections and to autoimmune diseases.
“Major histocompatibility complex” (MHC) proteins are found on the surfaces of most cells in vertebrate animals. They distinguish proteins like themselves from foreign proteins, and trigger an immune response against these foreign invaders.
MHCs recognize invading germs, reject or accept transplanted organs and play a role in helping vertebrates smell compatible mates.
“Results of this study explain why there are so many versions of the MHC genes, and why the ones that cause susceptibility to diseases are being maintained and not eliminated,” says biologist Wayne Potts of the University of Utah.
“They are involved in a never-ending ‘arms race’ that causes them, at any point in time, to be good against some infections but bad against other infections and autoimmune diseases.”
By allowing a disease virus to evolve rapidly in mice, Potts, Jason Kubinak and other University of Utah scientists produced new experimental evidence for the arms race between genes and germs–known technically as “antagonistic co-evolution.”
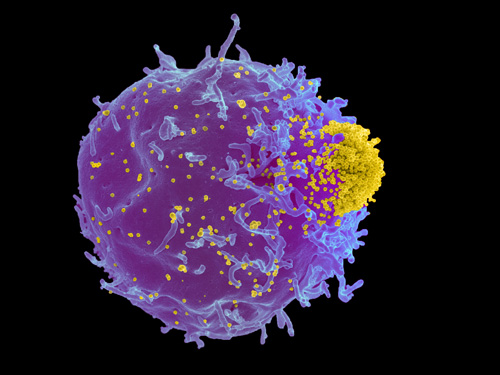
Friend mouse leukemia virus (yellow) budding from infected T-lymphocyte (blue). Image credit: Elizabeth Fischer and Kim Hasenkrug, NIH
The findings are published online this week in the journal Proceedings of the National Academy of Sciences (PNAS).
In addition to Potts and Kubinak, the paper’s lead author, the paper’s co-authors are James Ruff, Cornelius Whitney Hyzer, and Patricia Slev, all of the University of Utah.
The research was funded by the National Science Foundation (NSF) and the National Institute of Allergy and Infectious Diseases.
“The genetic diversity of the MHC complex is critical for vertebrates, including humans, to mount a defense against novel pathogens,” says George Gilchrist, acting deputy director of NSF’s Division of Environmental Biology, which funded the research.
“This study demonstrates that trade-offs between MHC genotypes and the severity of pathogen effects are key factors maintaining that diversity,” says Gilchrist. “The work has important implications for agricultural practice and conservation genetics, as well as human health.”
Most genes in humans and other vertebrates have only one or two “alleles,” varieties or variants of a single gene.
Although any given person carries no more than 12 varieties of the six human MHC genes, the human population has anywhere from hundreds to 2,300 varieties of each of the six human genes that produce MHC proteins.
“The mystery is why there are so many different versions of the same [MHC] genes in the human population,” Kubinak says, especially because many people carry MHCs that make them susceptible to pathogens (including the AIDS virus, malaria and hepatitis B and C) and autoimmune diseases (type I diabetes, rheumatoid arthritis, lupus, multiple sclerosis, irritable bowel disease and ankylosing spondylitis).
Scientists have proposed three theories for why so many MHC gene variants exist in vertebrate animal populations (invertebrates don’t have MHCs), and say all three likely are involved in maintaining the tremendous diversity of MHCs:
Theory one
An organism with more MHC varieties has a better immune response than organisms with fewer varieties, so over time, organisms with more MHCs are more likely to survive. However, this theory cannot explain the full extent of MHC diversity.
Theory two
Previous research indicates that people and other animals are attracted to the smell of potential mates with MHCs that are “foreign” rather than “self.” Parents with different MHC variants produce children with more MHCs and thus stronger immune systems.
Antagonistic co-evolution between an organism and its pathogens: “we have an organism and the microbes that infect it,” Kubinak says. “Microbes evolve to better exploit the organism, and the organism evolves better defenses to fight off the infection.” One theory to explain this great diversity in MHC genes is that those competing interests over time favor retaining more diversity.
“You naturally keep genes that fight disease,” says Kubinak. “They help you survive, so those MHC genes become more common in the population over time because the people who carry them live to have offspring.”
Theory three
Pathogens–disease-causing viruses, bacteria or parasites–infect animals, which defend themselves with MHCs that recognize the invader and trigger an immune response to destroy the invading pathogen.
But over time, some pathogens mutate and evolve to become less recognizable by the MHCs and thus evade an immune response.
As a result, the pathogens thrive.
MHCs that lose the battle to germs become less common because they now predispose people who carry them to become ill. It was thought that such disease-susceptibility MHC genes eventually should vanish from the population, but they usually don’t.
Why? While some of those MHCs do go extinct, others can persist, for two reasons.
First, some of the now-rare MHCs gain an advantage because they no longer are targeted by evolving microbes, so they regain an ability to detect and fight the same germ that earlier defeated them–after that germ mutates yet again.
Secondly, some of the rare MHCs can mount an effective immune response against completely different microbes.
The researchers studied 60 mice that were genetically identical, except that the mice were divided into three groups, each with a different variety of MHC genes known as b, d and k, respectively.
A mouse leukemia virus named the Friend virus was grown in tissue culture and used to infect two mice from each of the three MHC types.
The fast-evolving virus grew in the mice for 12 days, attacking, enlarging and replicating within the spleen and liver. Virus particles in the spleen were collected, and the severity of illness was measured by weighing the enlarged spleen.
Then, virus taken from each of the first three pairs of mice (b, d and k) was used to infect another three pairs of mice with the same MHC types.
The process was repeated until 10 pairs of mice in each MHC type were infected, allowing the virus time to mutate.
In this first experiment, the biologists showed that they could get the Friend virus to adapt to and thus evade the MHC variants (b, d or k) in the mouse cells it attacked.
Next, the researchers showed that the virus adapted only to specific MHC proteins. For example, viruses that adapted to and sickened mice with the MHC type b protein still were attacked effectively in mice that had the type d and k MHCs.
In the third experiment, the researchers showed that pathogen fitness (measured by the number of virus particles in the spleen) correlated with pathogen virulence (as measured by spleen enlargement and thus weight). So the virus that evaded the MHC type b made mice with that MHC sicker.
“The experiments demonstrate the first step in the antagonistic co-evolutionary dance between a virus and MHC genes,” Potts says.
The findings have important implications, say the scientists.
The use of antibiotics to boost productivity in dairy herds and other livestock is a major reason human diseases increasingly resist antibiotics. Selective breeding for more milk and beef has reduced genetic diversity in livestock, including their MHCs. So breeding more MHCs back into herds could enhance their resistance to disease and thus reduce the need for antibiotics.
Because their populations are diminished, endangered species have less genetic diversity, making them an easier target for germs. Potts says it would be desirable to breed protective MHCs back into endangered species to bolster their disease defenses.
Genetic variation of MHCs in people and other organisms is important for limiting the evolution and spread of emerging infectious diseases. In effect, the researchers created emerging diseases by making a virus evolve in mice. “It’s a model to identify what things change in viruses to make them more virulent,” says Potts, “and thus emerging diseases.”
*Source: National Science Foundation (NSF)
