Iron overload is a common condition affecting millions of people worldwide. Excess iron in the body is toxic, and deposits can cause damage to the liver, heart and other organs. Current treatments, researchers say, are not ideal and have significant side effects.
Iron in the body is regulated by a hormone called hepcidin, and a deficiency in this hormone can cause the iron overload seen in genetic disorders like hereditary hemochromatosis and Cooley’s anemia.
In the hopes of finding a treatment for iron overload, UCLA researchers have developed a new type of therapy based on small molecules that mimic the effects of hepcidin in mice. Published online Nov. 1 in the peer-reviewed Journal of Clinical Investigation, their findings could lead to new drugs to help prevent the condition.
Hepcidin works by fitting into a receptor protein known as ferroportin, which causes a change in iron flow in the body. The UCLA team systematically worked with the hormone–receptor interface to learn how the two pieces fit together and which part of hepcidin is the most important for binding to ferroportin.
“Like with jigsaw puzzle pieces, we tried to find the best fit,” said Dr. Elizabeta Nemeth, the study’s senior author and an associate professor of medicine at the David Geffen School of Medicine at UCLA.

Minihepcidin treatment prevents liver iron-loading in mice with hereditary hemochromatosis. The top panel shows a liver section from a mouse treated with placebo only, and the bottom panel shows a liver section from a mouse treated with minihepcidin. Blue color reveals iron deposits, pink color identifies cells with low iron content. Treatment with minihepcidin for two weeks prevented harmful accumulation of iron in the liver. Image credit: University of California
Nemeth, co-director of the UCLA Center for Iron Disorders, noted that this is the first attempt to develop medications that mimic hepcidin. Because hepcidin contains 25 amino acids and numerous disulfide bonds, it would be expensive and difficult to reproduce the hormone as a medication.
The UCLA team zeroed in on the areas of hepcidin and ferroportin that provided the best fit to generate iron-regulating activity. Surprisingly, they found that the first third of the hepcidin molecule had an effect similar to that of the whole molecule. They then re-engineered this portion of the molecule to make it even more effective and named the resulting new molecules “minihepcidins.”
“We found that just a few amino acids were enough to provide an effective scaffold for the minihepcidin design,” said Piotr Ruchala, a visiting assistant professor of medicine at the Geffen School of Medicine.
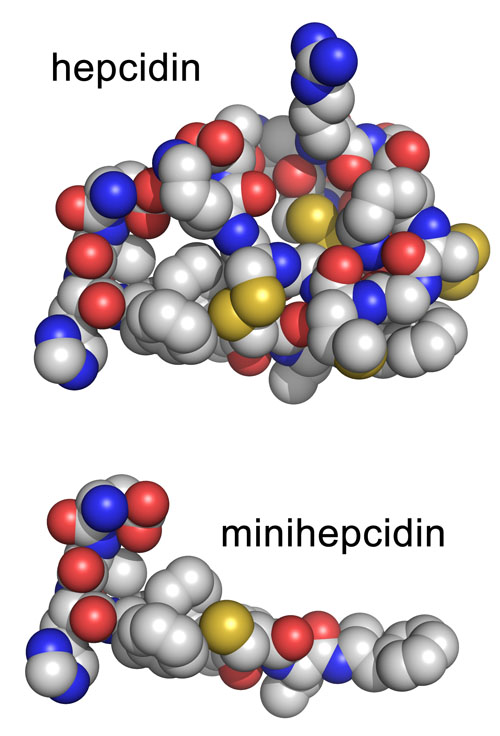
Structure of the human hormone hepcidin (top panel) and the portion used for the minihepcidin design (bottom panel). Image credit: University of California
The team confirmed that the minihepcidins were effective in healthy mice and demonstrated that they could prevent iron overload in mouse models of hereditary hemochromatosis.
“Using this structure and function analysis, we were able to develop minihepcidins that were even more effective than the naturally occurring hormone,” said study author Dr. Tomas Ganz, a professor of medicine and pathology and co-director of the Center for Iron Disorders at the Geffen School of Medicine.
Ganz added that the UCLA findings built on previous research by the team and collaborators around the world that originally helped identify the role of hepcidin and ferroportin in iron regulation.
The next step is to identify the optimal form of minihepcidin for human trials. According to UCLA researchers, if the molecules’ safety and efficacy is confirmed, minihepcidins could be used alone or together with current treatments for iron-overload diseases.
The study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases, which is part of the National Institutes of Health, and the Will Rogers Fund.
UCLA is currently negotiating a license to this technology with a biotechnology company that will take the minihepcidins through pre-clinical development and into clinical trials.
Other study authors included Gloria C. Preza of the UCLA Department of Pathology; Rogelio Pinon and Bo Qiao of the UCLA Department of Medicine; Emilio Ramos of the UCLA Department of Chemistry and Biochemistry; Michael Peralta of the Columbia University Department of Chemistry; Shantanu Sharma of the California Institute of Technology’s Materials and Process Simulation Center; and Alan Waring of the UC Irvine School of Medicine’s Department of Physiology and Biophysics.
– By Rachel Champeau
*Source: University of California
