An international group of scientists, including researchers from the Woods Hole Oceanographic Institution, are working to improve communication about ocean acidification to help the public better understand the pressing global issue.
The term “ocean acidification” (OA) describes the changes that occur in the ocean as a result of increased emissions of carbon dioxide (CO2) into the atmosphere. The rising acidic level in the ocean puts certain organisms at risk and threatens the overall health of the ocean.
Scientists have made advances in their understanding of the OA problem in recent years, propelling the group of OA researchers to coalesce the knowledge into a document understandable to specialist and non-specialists, alike.
The document is available at www.whoi.edu/OCB-OA/FAQs and in hard copy format at the Oceans in a High CO2 World Meeting, Monterey, CA, Sept. 24 – 27, 2012.

Ocean waters are becoming less and less welcoming for shelled organisms, says Sarah Cooley, a researcher in the WHOI Marine Chemistry and Geochemistry Department. Intensive burning of fossil fuels and deforestation over the last two centuries have increased carbon dioxide (CO2) levels in the atmosphere by almost 40 percent. The oceans absorb about one-third of all human-generated carbon emissions, but the buildup of CO2 in the ocean is pushing surface waters toward more acidic conditions, posing a threat to some marine organisms. (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)
Led by Ocean Carbon Biogeochemistry (OCB) and the UK Ocean Acidification Research Programme, the effort presents a summary of the state of knowledge on OA in FAQ or “frequently asked questions” format. Important for key communicators like journalists, policy specialists, educators, and the like, it explains the findings of recent research initiatives and puts the information in plain-language that can be used to educate or inform policy decision-making. Where possible, the degree of certainty in the knowledge is stated; where necessary, uncertainties are summarized.
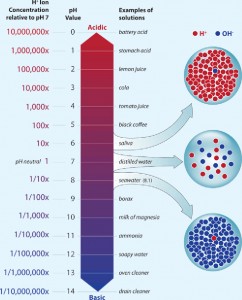
A pH of 7 is neutral, a pH less than 7 is acidic, and a pH greater than 7 is basic. Ocean water is normally slightly basic, with a surface-water pH of about 8.2, but that has declined in recent years to about 8.1. A decrease of 0.1 pH units may not seem like much, but because the pH scale is logarithmic, each unit on the pH scale represents a tenfold change in acidity. Image credit: Woods Hole Oceanographic Institution
“The science of ocean acidification is broad and fast moving, and keeping up with the literature can be a job unto itself. It’s crucial that the public have a clear understanding of this problem, if we are to have a hope of containing it or adapting to it,” said Sarah Cooley, a researcher in the WHOI Marine Chemistry & Geochemistry Department. “Much of the ocean acidification research happening now is funded by tax dollars, and people deserve to know why scientists are doing this work. Informing the public is also a great way to generate discussions of how human communities can make decisions about how to use of the Earth’s resources wisely while still providing opportunities for equitable human development.”
The release of this accumulated knowledge in an understandable format coincides with the Oceans in a High CO2 World in Monterey, CA, where scientists from around the world are gathered to share their research results and develop new research approaches to understanding and dealing with this problem. Communicators at the conference can use the new resource to integrate knowledge gained from scientific sessions.
The new document builds on FAQs first published in 2010, adding many new questions and updating answers to include new findings. The web version provides a three-level format for most questions with basic, intermediate, and advanced answers. Basic answers provide brief 2-3 sentence summaries of the more detailed intermediate and advanced answers. Intermediate answers target users with some scientific training, but no specialization. Advanced answers provide references to the primary literature.
The document is distributed via the Web at www.whoi.edu/OCB-OA/FAQs

Two tropical pencil urchins (Eucidaris tribuloides) reared under different levels of atmospheric carbon dioxide (CO2). The urchin on the right, grown in seawater under today's air conditions (400 ppm), is healthy and has normal spines. The urchin on the left, grown under very high CO2 conditions (2,850 ppm), is substantially damaged by the more acidic conditions. (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)
*Source: Woods Hole Oceanographic Institution
