Heart attacks during pregnancy are uncommon, but the prevalence of heart disease in pregnant mothers has increased over the past decade as more women delay pregnancy until they are older. These women, who are generally less physically active than their younger peers, tend to have higher cholesterol levels and are at greater risk of heart disease and diabetes.
While research has shown that the heart typically functions better during pregnancy due to a rise in cardiac pumping capacity to meet increased demands, a new UCLA study in rats and mice demonstrates that heart attacks occurring in the last trimester or late months of pregnancy result in worse heart function and more damaged heart tissue than heart attacks among non-pregnant females.
The research is published in the July edition of the peer-reviewed journal Basic Research in Cardiology.
“This very early study may help us identify and better understand the mechanisms involved in the higher risks of heart disease during pregnancy and may provide new opportunities to better treat pregnant women with cardiovascular complications and risk factors,” said senior study author Dr. Mansoureh Eghbali, an assistant professor of anesthesiology at the David Geffen School of Medicine at UCLA.
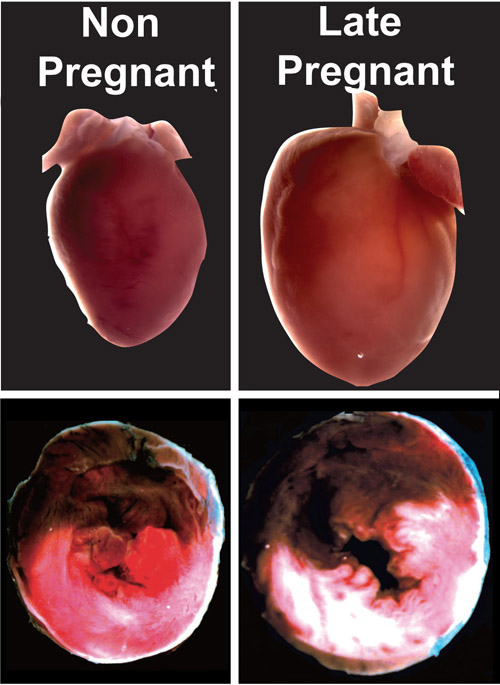
Heart attacks in late pregnancy. Top panels show the hearts of a non-pregnant and pregnant rat after a heart attack. Bottom panels show cross-sections of the hearts -- note the damaged areas of the hearts (shown in white) are much larger during the late months of pregnancy. Image credit: University of California
For the study, researchers assessed heart differences after heart attacks among late-stage pregnant female rats and non-pregnant animals. They found that the pregnant animals’ hearts demonstrated poor functional recovery, with only 10 percent restoration of heart function, compared with 80 percent restoration among the non-pregnant group. The pregnant animals also had a four-fold increase in damaged heart tissue over the non-pregnant group.
“We observed worse heart function and a greater area of damage in hearts from the late-pregnancy group, compared to the non-pregnant group,” said first author Dr. Jingyuan Li, a postdoctoral fellow in the department of anesthesiology at the Geffen School of Medicine.
“These findings show that the heart in late pregnancy may be particularly vulnerable to the type of injury caused by a heart attack,” Eghbali said.
Surprisingly, one day after giving birth, the formerly pregnant animals’ heart function was partially restored; after seven days, their heart function was almost fully restored to the levels of the corresponding non-pregnant state.
Researchers took a closer look to see what mechanisms were behind the heart’s inability to effectively recover after such a cardiac event in late pregnancy.
After a heart attack — caused by a lack of blood flow to the heart — the reintroduction of blood to the affected area can sometimes lead to problems, a phenomenon known as reperfusion injury, they said. When the blood hits the oxygen-starved tissue, it can result in a sudden increase in oxygen radicals that cause cell damage.
The research team found that in late pregnancy, several components of this process are especially aggravated, including the dysfunction of mitochondria, which are key cellular sub-units that are involved in cell death, and the reduction of signaling proteins that protect the heart against reperfusion injury.
Eghbali noted that the next stages of research will focus on understanding in more detail why the heart in late pregnancy is at a higher risk for coronary heart disease and will explore interventions and identify promising candidates for drug therapy.
The study was funded by the National Institutes of Health (grants HL089876, HL089876S1, HL077440 and HL088975).
Other study authors include Soban Umar, Andrea Iorga, Ji-Youn Youn, Yibin Wang and Hua Cai with the UCLA Department of Anesthesiology and the UCLA Cardiovascular Research Laboratories, and Vera Regitz-Zagrosek with the Institute of Gender in Medicine and the Center for Cardiovascular Research at Charité University Hospital in Berlin.
– By Rachel Champeau
*Source: University of California


